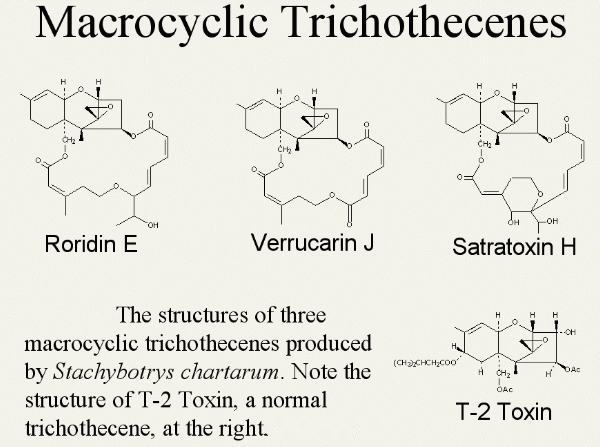
The trichothecenes are a large family of metabolites produced by several species of molds including Fusarium, Myrothecium, Trichoderma, Trichothecium, Cephalosporium, Verticimonosporium and Stachybotrys.
They are remarkably stable under different environmental conditions, including typical cooking temperatures. They consist of what is defined as mononocyclic (T-2 toxin) or macrocyclic (Satratoxin).
T-2 toxin is produced by several Fusarium spp. It is a contaminant of various cereal grains and is thought to be the major component of Yellow Rain of the Viet Nam era (Wannenmacher & Wiener, 1997).
The macrocyclic trichothecenes are produced by Stachybotrys chartarum (Satratoxins H and G, Roridin E, and Verrucarin J) (Bata et al, 1995).
The trichothecenes are nonvolatile with a molecular weight between 250-500. They are relatively insoluble in water, but highly soluble in a variety of solvents (acetone, ethyl acetate, DMSO, ethanol, methanol and propylene glycol).
Purified trichothecenes have a low vapor pressure and form a yellow color in solvents as well as a crystal. They are relatively stable compounds as noted above. They are not inactivated by autoclaving but require temperatures of 900 F 10 minutes or 500 F for 30 minutes for inactivation.
General Comments on the Toxicology of Trichothecenes
All trichothecenes are considered mycotoxins. They are toxic to humans, other mammals (domestic and research), birds, invertebrates, plants and eukaryote cells, in general. The acute toxicity of (LD50) to various species of animals has been reviewed by Wannenmacher and Wiener, 1997. They are more toxic via the lungs vs other means of exposure. For example, in the mouse, intranasal (0.6 mg/kg), intrathecal (0.01 mg/g) and inhalational (0.05 mg/kg) exposures are more toxic than intravenous (4.2-7.3 mg/kg) or intraperitoneal (5.2-9.1 mg/kg).
Acute Toxicity
Acute effects of oral, parenteral, dermal or aerosol exposure to trichothecenes produce a variety of effects: hematopoietic, radio mimetic, gastric an intestinal lesions and immune-suppression; neurotoxicity (nausea, anorexia, lassitude), suppression of reproduction function and vascular effects leading to hypotension. These effects occur because trichothecenes are potent inhibitors of protein synthesis. They bind to ribosomes, inhibiting protein and, subsequently, RNA and DNA synthesis.
Rapidly proliferating tissues (intestines and bone marrow) are most adversely affected. Furthermore, they are lipid soluble, crossing cell membranes, causing lipid peroxidation with mitochondrial and cellular membrane damage (for review, see Wannenmacher & Wiener, 1997).
Trichothecenes bind to subcellular structures, disrupting and altering the morphology of mitochondria, rough endoplasmic reticulum, myofibers and membranes (Yarom & More, 1983; Trusal & O’Brien, 1986).They inhibit succinic dehydrogenase activity with effects on cellular energetics with decreases in succinate, pyruvate and malate oxidation and inhibition of mitochondrial protein synthesis (Lauren & Smith, 1989; Pace et al, 1988; Pace, 1983).
Finally, they cause increased cell death (apoptosis) in a variety of cell types via mitochondrial and non-mitochondrial mechanisms (Ishigami et al, 2001; Yang et al, 2000; Nagase et al, 2001, 2002; Poapolathep et al, 2002; Shifrin & Anderson, 1999).
Furthermore, trichothecenes readily cross the placenta and have been shown to cause increased cell death (apoptosis) in mouse fetuses (Ishigami et al, 2001).
It is suspected that from 1974 to 1981, trichothecenes were used in Afghanistan, Laos and Cambodia via aerial application ("yellow rain") (Wannenmacher & Wiener, 1997; Tucker, 2002). Early symptoms in "yellow rain" victims were severe nausea, vomiting, burning superficial skin discomfort, lethargy, weakness, dizziness and loss of coordination.
Within minutes to hours, diarrhea (first watery brown and later grossly bloody) occurred. From 3 to 12 hours, symptoms included dyspnea, coughing, sore mouth, bleeding gums, epistaxis, hematemesis, abdominal pain and central chest pain. Exposed skin could become red, tender, swollen, painful or pruritic. Small or large vesicles and bullae were observed as well as petechiae, ecchymosis and necrosis of the skin. Marked anorexia and dehydration were frequent.
Dying individuals became hypothermic, hypotensive and developed tachycardia. Severely poisoned individuals had bloody ooze from the nose and mouth with an associated hematochezia. Death occurred from minutes to hours and days and was often preceded by tremors, seizures and coma. The most frequent symptoms included vomiting (71%), diarrhea (53%), skin irritation, burning and itching (44%), rash or blisters (33%), bleeding (53%) and dyspnea (48%). All of the symptoms listed could be attributed to trichothecene toxicity (Wannenmacher & Wiener, 1997).
Wannenmacher and Wiener (1997) reported ocular and respiratory findings. Both ocular and upper respiratory effects were observed with the "yellow rain" exposure. Ocular signs and symptoms were tearing, pain, conjunctivitis and burning sensations about the eyes. These lasted from 8 to 14 days following exposure. Of interest, a trichothecene (DAS) isolated from one autopsy case instilled into the eyes of rabbits produced reddening, edema and cornea opacity.
Upper respiratory symptoms included the following: nose (itching, pain rhinorrhea, epistaxis), throat (sore/pain, aphona, voice changes) and tracheobronchial tree (cough, hemoptysis, dyspnea, deep chest pain, chest pressure).
Agricultural workers exposed to hay or hay dust contaminated with Trichothecenes also developed similar signs and symptoms of upper respiratory injury.
Fetal Effects
The administration of T-2 toxin to pregnant rats and mice has resulted in adverse effects in the placenta and fetus. Cell death (apoptosis) occurs in the placenta and fetal liver resulting from reactive oxygen species via induction of mitogen-activated protein kinase (MAPK) (Sehata et al, 2004, 2005). In mice, T-2 toxin crosses the placenta barrier causing atrophy of the fetal thymus as a result of apoptosis of lymphocyte precursor cells (Ishigami et al, 2001; Holladay et al, 1993).
Chronic Toxicological Effects
Chronic exposure to trichothecenes causes Alimentary Toxic Aleukia (ATA) in humans, mycotoxicosis in domestic animals and adverse outcomes in individuals given trichothecenes intravenously as a chemotherapy for colon adenocarcinoma (for review, see Wannenmacher & Wiener, 1997).
ATA occurred in Russia during and prior to WW II when peasants consumed field grains contaminated with trichothecene mycotoxins infested with Fusarium. The clinical course of the disease occurred in four stages (Joffe, 1971).
Stage one was characterized by inflammation of the gastrointestinal tract mucosa, vomiting, diarrhea, abdominal pain, excessive salivation, headache, dizziness, weakness, fatigue, tachycardia, fever and sweating.
Progression occurs to the second stage (also called leukopenic or latent stage). Leukopenia, granulopenia and progressive lymphocytosis characterize this stage. If ingestion of the contaminated grain is not stopped or if a large dose is taken in, the third stage ensues.
The third stage is characterized by a bright red or dark cherry-red, petechial rash on the chest and other areas of the body. These are at first localized and then spread, becoming more numerous. In the most severe cases, intensive ulceration and gangrenous conditions develop in the larynx. This can lead to aphonia and death by strangulation. Concomitantly, hemorrhagic diathesis occurs in the nasal, oral, gastric and intestinal mucosa.
The fourth stage (recovery stage) begins when the necrotic lesions of the body begin to heal and the body temperature drops. The affected individuals are susceptible to secondary infections, including pneumonia. Convalescence takes several weeks and the bone marrow approaches normality by two months.
Chemotherapy
The trichothecenes inhibit cell division via cell death. This was used as a basis for a chemotherapy drug trial (Claridge et al, 1979; Goodwin et al, 1979; Murphy et al, 1978). Cancer patients were given daily doses (0.077 mg/kg) of DAS (anguidine) for 5 days. They developed signs and symptoms of toxicity which included nausea, vomiting, diarrhea, burning erythema, confusion, ataxia, chills, fever, hypotension and hair loss. The anti-tumor activity was either absent or minimal and the drug trials were stopped because of patient intolerance.
Metabolism
Trichothecenes, unlike other mycotoxins, do not require metabolic activation to exert their toxic effects (Busby & Wogan, 1981). Direct dermal application leads to immediate skin irritation.
Trichothecenes directly act with cellular organelles and structures causing inhibition of protein, RNA and DNA synthesis, disaggregation of polyribosomes and rough endoplasmic reticulum, inhibition of mitochondrial functions and cause cell death (apoptosis). (Yarom et al, 1983; Trusal & O’Brien, 1986; Pace et al, 1983, 1988; Ishigami et al, 2001; Yang et al, 2000; Nagase et al, 2001, 2002; Poapolathep et al, 2002; Shifrin & Anderson, 1999’ Joffe, 1971; Busby & Wogan, 1981; McLaughlin et al, 1977; Trusal, 1985; Leatheman & Middlebrook, 1993).
Trichothecenes are lipophilic and are easily absorbed through the skin, respiratory and intestinal tracts. A single oral dose peaks in the blood at one hour. Inhaled median lethal dose is equal to or less than a systemic dose.
Death in rodents and guinea pigs occurs in 1 to 12 hours, following inhalation of lethal aerosol concentrations without pulmonary edema (Joffe, 1971).
Tissue distribution studies show that the liver is the major organ of metabolism of trichothecenes.
Radioactivity from labeled mycotoxins following different routes of administration (oral, intra muscular, IV, dermal) appear in the bile, liver and gastrointestinal tract with metabolites and glucuronide conjugates appearing in the urine and feces (Matsumoto et al, 1978; Corley et al, 1985).
Trichothecenes are metabolized via deacetylation and de-expoxidation (hyrdrolysis). The metabolic fate of T-2 toxin has been the most thoroughly investigated of all of the trichothecenes. It is metabolized by rat intestinal microflora in a variety of animals to de-epoxy products (DE HT-2 and DE TRIOL).
Also, DAS is bio-transformed by de-acetylation and de-epoxidation by intestinal microflora of cattle, swine and rats (Swanson et al, 1988). A nonspecific carboxylesterase in the liver selectively hydrolyzes the C-4 acetyl group of T-2 toxin to form HT-2 toxin (Johnsen et al, 1986). The activity of this enzyme has also been detected in the brain, kidney, spleen, white blood cells and erythrocytes (Wannenmacher & Wiener, 1997; Ohta et al, 1977).
Also, a hepatic cytochrome P-450 in mice and monkeys has been shown to catalyze the hydrolysis of the C-3' and C-4' positions of the isovaleryl side chain of T-2 and HT-2 toxins (Yoshizawa et al, 1984; Kohbayashi et al, 1987).
Finally, it is of interest to note that chronic exposure to 6-12 ppm of trichothecenes in the diet causes an increase in drug metabolizing enzymes, while acute low doses produces a decrease in these microsomal enzymes (Yabe et al, 1993; Galtier et al, 1989; Guerre et al, 2000).
The subcellular distribution of tritium labeled T-2 toxin has been reported in perfused isolated rat livers. After 120 minutes of perfusion, the distribution of the radiolabel was bile (53%), perfusate (39%) and liver (7%). The radiolabel within subcellular fractions was located in the plasma membrane, and smooth endoplasmic reticulum within 5 minutes, declining thereafter. The uptake in mitochondria occurred in 30 minutes and increased by 120 minutes after perfusion. The labeling in the nucleus remained constant throughout the 120 minutes. The time course for distribution of labeled T-2 showed an immediate association with plasma membranes and a subsequent distribution of the toxin and metabolites with endoplasmic reticulum, mitochondria and nuclei -- the known sites of action of this toxin (Pace & Watts, 1989).